Torsional chirality and molecular recognition: the homo and heterochiral dimers of thenyl and furfuryl alcohol†
Abstract
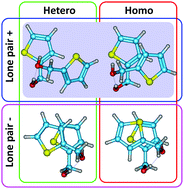
Furfuryl alcohol and thenyl alcohol contain a labile torsional chiral center, producing transiently chiral enantiomers interconverting in the nanosecond time-scale. We explored chiral molecular recognition using the weakly-bound intermolecular dimers of both alcohols, freezing stereomutation. Supersonic jet broadband microwave spectroscopy revealed homo and heterochiral diastereoisomers for each alcohol dimer and the structural characteristics of the clusters. All dimers are primarily stabilized by a moderately intense O–H⋯O hydrogen bond, but differ in the secondary interactions, which introduce additional hydrogen bonds either to the ring oxygen in furfuryl alcohol or to the π ring system in thenyl alcohol. Density-functional calculations (B2PLYP-D3(BJ)/def2-TZVP) show no clear preferences for a particular stereochemistry in the dimers, with relative energies of the order 1–2 kJ mol−1. The study suggests opportunities for the investigation of chiral recognition in molecules with torsional barriers in between transient and permanent interconversion regimes.


 Please wait while we load your content...
Please wait while we load your content...