Flavin-mediated photoactivation of Pt(iv) anticancer complexes: computational insights on the catalytic mechanism†
Abstract
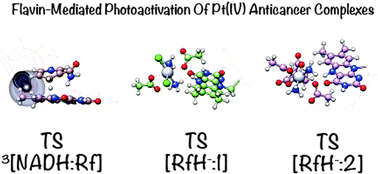
The mechanism for the photocatalytic activation of Pt(IV) anticancer prodrugs by riboflavin in the presence of NADH has been investigated by DFT. In the first step of the reaction, the oxidation kinetics of NADH to afford the catalytically active riboflavin hydroquinone is dramatically favoured by generation of the flavin triplet excited state. In the triplet, formation of a π–π stacked adduct promotes the hydride transfer from NADH to riboflavin with an almost barrierless pathway (2.7 kcal mol−1). In the singlet channel, conversely, the process is endergonic and requires overcoming a higher activation energy (19.2 kcal mol−1). In the second half of the reaction, the reduction of the studied Pt(IV) complexes by riboflavin hydroquinone occurs via an inner sphere mechanism, displaying free energy barriers smaller than 10 kcal mol−1. Pt reduction by bioreductants such as NADH and ascorbate involve instead less stabilized transition states (22.2–38.3 kcal mol−1), suggesting that riboflavin hydroquinone is an efficient reducing agent for Pt(IV) derivatives in biological settings.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...