Concentration-dependent ion correlations impact the electrochemical behavior of calcium battery electrolytes†
Abstract
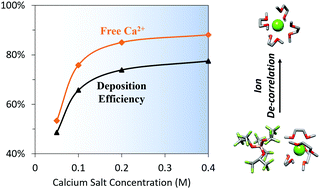
Ion interactions strongly determine the solvation environments of multivalent electrolytes even at concentrations below that required for practical battery-based energy storage. This statement is particularly true of electrolytes utilizing ethereal solvents due to their low dielectric constants. These solvents are among the most commonly used for multivalent batteries based on reactive metals (Mg, Ca) due to their reductive stability. Recent developments in multivalent electrolyte design have produced a variety of new salts for Mg2+ and Ca2+ that test the limits of weak coordination strength and oxidative stability. Such electrolytes have great potential for enabling full-cell cycling of batteries based on these working ions. However, the ion interactions in these electrolytes exhibit significant and non-intuitive concentration relationships. In this work, we investigate a promising exemplar, calcium tetrakis(hexafluoroisopropoxy)borate (Ca(BHFIP)2), in the ethereal solvents 1,2-dimethoxyethane (DME) and tetrahydrofuran (THF) across a concentration range of several orders of magnitude. Surprisingly, we find that effective salt dissociation is lower at relatively dilute concentrations (e.g. 0.01 M) than at higher concentrations (e.g. 0.2 M). Combined experimental and computational dielectric and X-ray spectroscopic analyses of the changes occurring in the Ca2+ solvation environment across these concentration regimes reveals a progressive transition from well-defined solvent-separated ion pairs to de-correlated free ions. This transition in ion correlation results in improvements in both conductivity and calcium cycling stability with increased salt concentration. Comparison with previous findings involving more strongly associating salts highlights the generality of this phenomenon, leading to important insight into controlling ion interactions in ether-based multivalent battery electrolytes.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...
