Unraveling the acid–base characterization and solvent effects on the structural and electronic properties of a bis-bidentate bridging ligand†
Abstract
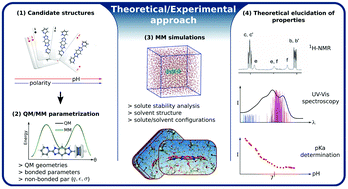
Understanding the interactions and the solvent effects on the distribution of several species in equilibrium and how it can influence the 1H-NMR properties, spectroscopy (UV-vis absorption), and the acid–base equilibria can be especially challenging. This is the case of a bis-bidentate bridging ligand bis(2-pyridyl)-benzo-bis(imidazole), where the two pyridyl and four imidazolyl nitrogen atoms can be protonated in different ways, depending on the solvent, generating many isomeric/tautomeric species. Herein, we report a combined theoretical–experimental approach based on a sequential quantum mechanics/molecular mechanics procedure that was successfully applied to describe in detail the acid–base characterization and its effects on the electronic properties of such a molecule in solution. The calculated free-energies allowed the identification of the main species present in solution as a function of the solvent polarity, and its effects on the magnetic shielding of protons (1H-NMR chemical shifts), the UV-vis absorption spectra, and the acid–base equilibrium constants (pKas) in aqueous solution. Three acid–base equilibrium constants were experimentally/theoretically determined (pKa1 = 1.3/1.2, pKa2 = 2.1/2.2 and pKa5 = 10.1/11.3) involving mono-deprotonated and mono-protonated cis and trans species. Interestingly, other processes with pKa3 = 3.7 and pKa4 = 6.0 were also experimentally determined and assigned to the protonation and deprotonation of dimeric species. The dimerization of the most stable neutral species was investigated by Monte Carlo simulations and its electronic effects were considered for the elucidation of the UV-vis absorption bands, revealing transitions mainly with the charge-transfer characteristic and involving both the monomeric species and the dimeric species. The good matching of the theoretical and experimental results provides an atomistic insight into the solvent effects on the electronic properties of this bis-bidentate bridging ligand.


 Please wait while we load your content...
Please wait while we load your content...