Investigation of the role of hydrogen bonding in ionic liquid-like salts with both N- and S-soft donors†
Abstract
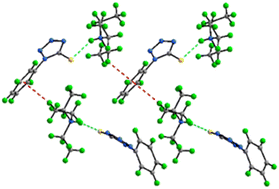
In search of ionic liquids (ILs) with multiple types of soft donor atoms capable of preferentially complexing a range of soft metal ions over harder ions, we investigated structural clues to the role of hydrogen bonding in IL behavior through a series of salts with anions containing both N- and S-donor atoms based on azole thiolates. Reaction of equimolar amounts of triethylamine (Et3N) or diisobutylamine (DBA) with 1-phenyl-1H-tetrazole-5-thiol (PhTzSH), 1-methyl-1H-tetrazole-5-thiol (MeTzSH), or 5-methyl-1,3,4-dithiazole-2-thiol (MeDiTSH) yielded [Et3NH][MeTzS] (1), a yellow liquid, and the low melting yellow solids [DBAH][MeTzS] (2), [Et3NH][PhTzS] (3), [DBAH][PhTzS] (4), [Et3NH][MeDiTS] (5), and [DBAH][MeDiTS] (6). Thermal analysis revealed that all of them qualify as ILs with melting points below 100 °C. Single crystal X-ray structure analysis of 2–6 revealed the presence of an extensive H-bonding network that includes the rare N–H⋯S hydrogen bonds in 3, 4, and 6. These weaker interactions appear to significantly influence thermal behavior, where strong bonding leads to higher melting temperatures and lower decomposition points.


 Please wait while we load your content...
Please wait while we load your content...