Quasi-aromatic Möbius chelates of cadmium(ii) nitrite and/or nitrate†
Abstract
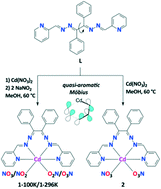
We report the design, structural, spectroscopic and computational characterization of the two new quasi-aromatic Möbius chelate coordination compounds fabricated from Cd(NO3)2·4H2O and a bulky helical organic ligand derived from benzildihydrazone and 2-pyridinecarboxaldehyde (L) in the presence and absence of two equivalents of NaNO2, namely [Cd(NO2)x(NO3)2−x(L)]·2MeOH (1·2MeOH) and [Cd(NO3)2(L)]·MeOH (2·MeOH). The former complex was found to extensively lose one of the lattice solvent molecules of methanol under ambient conditions upon isolation from the mother liquor. Therefore, the crystal structure of 1·2MeOH was obtained at 100 K, namely [Cd(NO2)1.67(NO3)0.33(L)]·2MeOH ((1-100 K)·2MeOH), and at 296 K, namely [Cd(NO2)1.57(NO3)0.43(L)]·2MeOH ((1-296 K)·MeOH). In all the structures, anions exhibit a chelate κ2-O,O-mode. It was found that the reported Cd–NO2 and Cd–NO3 connections are largely ionic in nature, followed by dative covalent charge delocalization and the London dispersion constituents. The electron density of delocalized bonds (EDDB) function and the extended transition state (ETS) energy decomposition method combined with the natural orbitals for chemical valence (ETS-NOCV) have shown sizeable π-delocalizations within the twisted ligand L suggesting quasi-aromatic Möbius features of the Cd–L chelate. Finally, the optical properties of (1-296 K)·MeOH and 2·MeOH have been investigated by diffuse reflectance spectroscopy.


 Please wait while we load your content...
Please wait while we load your content...