Abstract
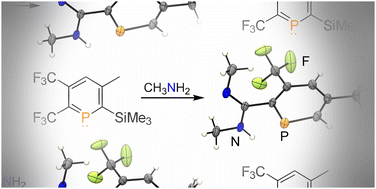
An unexpected route to hitherto unknown amidine-functionalized phosphinines has been developed that is rapid and simple. Starting from primary amines and CF3-substituted λ3,σ2-phosphinines, a cascade of dehydrofluorination reactions leads selectively to ortho-amidinephosphinines. DFT calculations reveal that this unusual transformation can take place via a series of nucleophilic attacks at the electrophilic, low-coordinate phosphorus atom.


 Please wait while we load your content...
Please wait while we load your content...