TfOH-promoted synthesis of indoles and benzofurans involving cyclative transposition of vinyl ketone†
Abstract
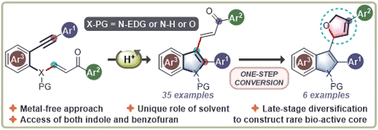
A metal-free approach to construct indole rings from vinylogous amides derived from o-alkynylanilines involving a cyclization, retro-aza-Michael reaction and amine trapping cascade is reported here. This atom-economical transformation has been extended to synthesize benzofuran derivatives using analogous vinylogous esters derived from o-alkynylphenols. The excellent stereochemical outcome of the double bond geometry in the products makes it attractive.


 Please wait while we load your content...
Please wait while we load your content...