A new access to diazaphospholes via cycloaddition–cycloreversion reactions on triazaphospholes†
Abstract
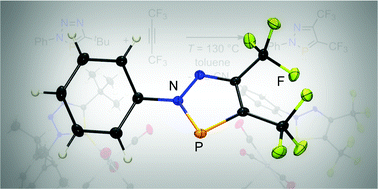
A novel bis-CF3-substituted diazaphosphole was synthesized selectively from hexafluoro-2-butyne and a 3H-1,2,3,4-triazaphosphole derivative. The [4+2] cycloaddition and subsequent cycloreversion reaction under elimination of pivaloyl nitrile affords the product in high yield. The heterocycle coordinates via the phosphorus atom to a W(CO)5-fragment and shows stronger π-accepting properties than the triazaphosphole.
- This article is part of the themed collection: Chemical Communications HOT Articles 2022


 Please wait while we load your content...
Please wait while we load your content...