Exploiting the umpolung reactivity of diazo groups: direct access to triazolyl-azaarenes from azaarenes†
Abstract
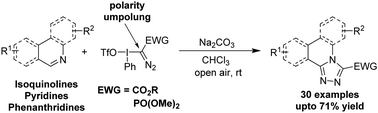
The present work documents electrophilic substitution of azaarenes, mainly isoquinolines, with hypervalent iodine diazo reagents (HIDR) followed by formal [3+2]-dipolar cycloaddition in a tandem fashion. Other azaarenes viz. pyridines and phenanthridines too could be successfully used in the reaction. The methodology capitalizes on the umpolung nature of α-aryliodonio diazo compounds for installing a nucleophile, i.e. azaarene, at their α-position. Subsequent ylide formation and intramolecular 1,5-cyclization furnished 4,3-fused 1,2,4-triazolyl-azaarenes in good yields. The reaction is notable for its mild conditions, operational simplicity and fairly general scope.


 Please wait while we load your content...
Please wait while we load your content...