Extension of hydrogen borrowing alkylation reactions for the total synthesis of (−)-γ-lycorane†
Abstract
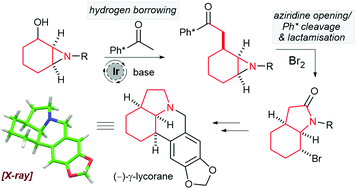
The total synthesis of (–)-γ-lycorane (10 steps) and synthesis of (±)-γ-lycorane (8 steps) was completed from cyclohexenone. A new two step hydrogen borrowing alkylation of an aziridinyl alcohol, coupled with a Ph* (Me5C6) deprotection/cyclisation procedure was developed for de novo formation of the fused 6,5 heterocyclic ring. This work is one of the first examples of hydrogen borrowing C–C bond formation being used as a key step in a total synthesis project.
- This article is part of the themed collection: Chemical Communications HOT Articles 2022


 Please wait while we load your content...
Please wait while we load your content...