Titanium isopropoxide-mediated cis-selective synthesis of 3,4-substituted butyrolactones from CO2†
Abstract
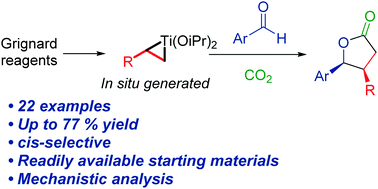
We report a Ti(OiPr)4-mediated multicomponent reaction, which produces 3,4-substituted cis-δ-lactones from alkyl magnesium chloride, benzaldehyde and CO2. The key intermediate, titanacyclopropane, is formed in situ from Ti(OiPr)4 and a Grignard reagent, which enables 1,2-dinucleophilic reactivity that is used to insert carbon dioxide and an aldehyde. An alternative reaction route is also described where a primary alkene is used to create the titanacyclopropane. A computational analysis of the elementary steps shows that the carbon dioxide and the aldehyde insertion proceeds through an inner-sphere mechanism. A variety of cis-butyrolactones can be synthesized with up to 7 : 1 diastereoselectivity and 77% yield.


 Please wait while we load your content...
Please wait while we load your content...