Copper(i) activation of C–X bonds: bimolecular vs. unimolecular reaction mechanism†
Abstract
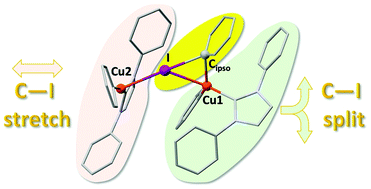
Experimental kinetic studies and DFT calculations show that the oxidative addition of aryl halides (Ar–X) to complexes [Cu(NHC)R] follow different paths depending on the nature of X. For X = Br a concerted addition leads to cis-[Cu(NHC)XRAr] from which the usual C–C coupled product Ar–R eliminates. However, for X = I trans-[Cu(NHC)IRAr] is formed instead, leading to the elimination of R–I in a metathesis reaction. This behaviour is accounted for by a change in the reaction mechanism for Ar–I, which involves two molecules of copper(I) complex, the second one stabilising the incipient iodide formed in the C–I breaking (oxidative addition) and C–I forming (reductive elimination) processes.


 Please wait while we load your content...
Please wait while we load your content...