Remote ortho-C–H functionalization via medium-sized cyclopalladation
Abstract
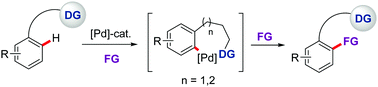
Compared to the tremendous progress made in directed ortho-C–H functionalization via five- or six-membered cyclopalladation, protocols with the ability to selectively activate more remote C–H bonds through the intermediacy of larger, less favorable, seven- or eight-membered metalacycles are particularly challenging and remain rare. However, such a strategy would provide new retrosynthetic opportunities for generating structural diversity and complexity. Intense recent research based on the use of either mono-anionic bidentate or monodentate directing groups is characterizing this approach as an increasingly viable tool for selective C–C and C–X bond-forming reactions. This short review provides an overview of these strategies with an emphasis on mechanistic details, synthetic applicability, limitations, and key challenges.


 Please wait while we load your content...
Please wait while we load your content...