De-sialylation of glycopeptides by acid treatment: enhancing sialic acid removal without reducing the identification†
Abstract
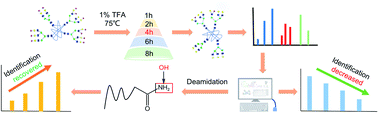
Sialic acid, a common terminal monosaccharide on many glycoconjugates, plays essential roles in many biological processes such as immune responses, pathogen recognition, and cancer development. For various purposes, sialic acids may need to be removed from glycopeptides or glycans, mainly using enzymatical or chemical approaches. In this study, we found that most commonly used chemical methods couldn't completely remove sialic acids from glycopeptides. Although the de-sialylation efficiency could be further enhanced by increasing the treatment time or acid concentration, the undesirable side reactions on the peptide portion would decrease glycopeptide identification. By adding the deamidation on carbamidomethyl-cysteine (C), asparagine (N), and glutamine (Q) residues as a variable modification during database search, most of the unidentified spectra could be recovered. This optional acid-treatment and database search method for the complete removal of sialic acids without losing much spectral identification should be quite useful for many glycomic and glycoproteomic studies.


 Please wait while we load your content...
Please wait while we load your content...