Heterostructured ferroelectric BaTiO3@MOF-Fe/Co electrocatalysts for efficient oxygen evolution reaction†
Abstract
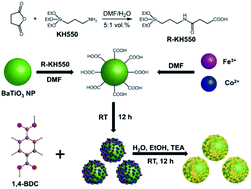
The ability to substitute expensive and scarce noble metals with cheap and readily accessible nanomaterials represents an important endeavor toward water electrolysis to produce hydrogen, an alternative green fuel. Herein, we report on the crafting of heterostructured electrocatalysts that integrate semiconducting two-dimensional (2D) bimetallic metal–organic framework nanoplatelets (i.e., MOF-Fe/Co, where Fe/Co = 1/2) and ferroelectric nanoparticles (NPs) with high dielectric constant (κ ∼ 270 for BaTiO3 NPs) for high-efficiency oxygen evolution reaction (OER). The OER activity of the BaTiO3@MOF-Fe/Co heterostructures was tested under a basic condition (1.0 M KOH), demonstrating an overpotential of 247 mV at 10 mA cm−2, a Tafel slope of 38.4 mV dec−1, and a double-layer capacitance (Cdl) of 85.6 mF cm−2, which outperformed the neat MOF-Fe/Co (i.e., 281 mV, 72.1 mV dec−1, and 37.3 mF cm−2, respectively). The density functional theory (DFT) calculation revealed that the effective electronic modulation between tetragonal BaTiO3 NP and MOF-Fe/Co nanoplatelets induced an apparent reduction in the free energy barrier for the transformation from O* to OOH* and desorption of O2. More importantly, both experimental and DFT results corroborated that the self-polarizing ferroelectric BaTiO3 NPs with a high dielectric constant generated a favorable local electric field and enhanced the charge conductivity of the MOF-Fe/Co moieties in the heterostructure, leading to a superior OER catalytic activity. This work not only presents a simple yet robust route to creating heterojunctioned nanostructures for highly efficient OER, but also provides a unique platform for designing superior, noble metal-free OER electrocatalysts via judiciously incorporating ferroelectric, high-κ nanomaterials.


 Please wait while we load your content...
Please wait while we load your content...