Tunable biomaterials for myocardial tissue regeneration: promising new strategies for advanced biointerface control and improved therapeutic outcomes†
Abstract
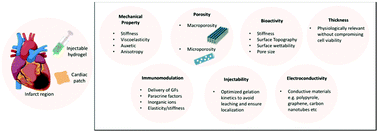
Following myocardial infarction (MI) and the natural healing process, the cardiac mechanostructure changes significantly leading to reduced contractile ability and resulting in additional pressure on the heart muscle thereby increasing the risk of heart failure (HF). The application of cardiac scaffolds in the form of epicardial patches or injectable hydrogels at the infarcted region of the myocardium helps to mechanically reinforce the ventricular walls and allows control over the various stages of the healing process, reducing pathological remodeling and fibrosis and eventually restoring cardiac function. Recent progress in the field of biomaterials engineering allows tuning of cardiac scaffold properties for more effective tissue-biomaterial interaction leading to improved therapeutic outcomes. Nanoscaffold characteristics required for myocardial tissue engineering (TE) including mechanical property, pore size/porosity, immunomodulation, bioactivity, electroconductivity, injectability (for hydrogels) and thickness (for cardiac patches) are herewith reviewed. Strategies for controlling each of these properties via blending, scaffold fabrication, degree of crosslinking, and incorporation of bioactive molecules, amongst others are also discussed. The mechanism of myocardial restoration via enhanced angiogenesis, stem cell homing and mechanical support is further detailed. Finally, key novel innovative strategies with high promise for clinical translation are presented; in particular, the use of extracellular vesicle-loaded scaffolds, integration of electronics within scaffolds for real time monitoring of the engineered tissue performance as well as the possibility of refilling scaffolds with drugs/cells/proteins via a subcutaneous port are highlighted.


 Please wait while we load your content...
Please wait while we load your content...