Constitutional isomers of carbazole–benzoyl-pyrimidine-based thermally activated delayed fluorescence emitters for efficient OLEDs†
Abstract
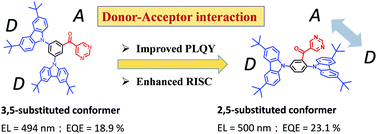
Thermally activated delayed fluorescence (TADF) emitters are highly useful to achieve 100% internal quantum efficiency (IQE) and high external quantum efficiency (EQE) by reverse intersystem crossing. Herein, four sky-blue to blueish-green TADF dopants, 35CzBPym, 35tCzBPym, 25CzBPym, and 25tCzBPym, containing benzoyl pyrimidine (BPym) as the acceptor and carbazoles (Czs) as the donors were designed, synthesized, and investigated. The as-prepared 35CzBPym and 25CzBPym isomers showed star and Y-shaped configurations as confirmed by a single-crystal X-ray diffractometer. Interestingly, Y-shaped emitters exhibit high photoluminescence quantum yields (PLQYs) (76–88%) together with small ΔEST (45–90 meV) values, leading to very efficient TADF. The strong intramolecular interactions between the nearby pyrimidine and carbazole planes and hydrogen bonds are crucial for enhancing molecular rigidity resulting in the high PLQY. Thus, an electroluminescent device based on 25tCzBPym shows bluish-green emission (500 nm) with a maximum EQE of 23.3% and a low-efficiency roll-off at high luminance of 1000 cd m−2 (28%). Moreover, the present molecular design exhibits combined effects of intramolecular charge transfer, donor–acceptor interactions, and hydrogen bonding within the molecular structures, supported by the DFT calculations and single-crystal analyses.
- This article is part of the themed collections: Journal of Materials Chemistry C Lunar New Year collection 2022 and 2021 Journal of Materials Chemistry C most popular articles


 Please wait while we load your content...
Please wait while we load your content...