Sensing and electrocatalytic activity of tungsten disulphide thin films fabricated via metal–organic chemical vapour deposition†
Abstract
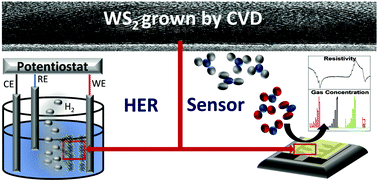
The unique structural and electronic properties of transition metal dichalcogenides (TMDs) and in particular tungsten disulphide (WS2) make them interesting for a variety of applications such as the electrocatalytic hydrogen evolution reaction (HER) for water splitting devices and chemiresistive gas sensors. The key parameter for the realisation of these devices is the controlled large-area growth of WS2 combined with tuning the surface morphology and electronic properties which is achieved by bottom-up fabrication methods such as chemical vapour deposition (CVD). In this study, 2H-WS2 films are fabricated by a new metal–organic CVD (MOCVD) process resulting in the growth of crystalline, pure, and stoichiometric films which was accomplished at temperatures as low as 600 °C as confirmed by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), Rutherford backscattering spectrometry (RBS)/nuclear reaction analysis (NRA), and Raman spectroscopy. The surface morphology of WS2 layers was investigated by scanning electron microscopy (SEM) and high-resolution transmission electron microscopy (HR-TEM). Following successful process development, the WS2 layers were deposited on conducting FTO/glass substrates and their catalytic activity for the HER was evaluated in a linear sweep voltammetry (LSV) experiment. Furthermore, the temperature-dependent sensor response towards NO2, CO, and NH3 was investigated for WS2 films deposited on special sensor chips, revealing a p-type response towards NO2 and NH3 and sensitivities of around 20% for NO2 and NH3 concentrations of 1.5 ppm and 7.6 ppm, respectively. These promising results demonstrate the effectiveness of scalable CVD-grown WS2 and pave the way for practical applications by modulating the properties of materials to achieve enhanced electrocatalytic and sensing performances employing WS2 layers.


 Please wait while we load your content...
Please wait while we load your content...