Interplay between ferroelectricity and metallicity in BaTiO3†
Abstract
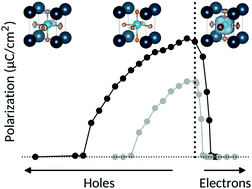
We explore the interplay between ferroelectricity and metallicity, which are generally considered to be contra-indicated properties, in the prototypical ferroelectric barium titanate, BaTiO3. Using first-principles density functional theory, we calculate the effects of electron and hole doping, first by introducing a hypothetical background charge, and second through the introduction of explicit impurities (La, Nb and V for electron doping, and K, Al and Sc for hole doping). We find that, apart from a surprising increase in polarization at small hole concentrations, both charge-carrier types decrease the tendency towards ferroelectricity, with the strength of the polarization suppression, which is different for electrons and holes, determined by the detailed structure of the conduction and valence bands. Doping with impurity atoms increases the complexity and allows us to identify three factors that influence the ferroelectricity: structural effects arising largely from the size of the impurity ion, electronic effects from the introduction of charge carriers, and changes in unit-cell volume and shape. A competing balance between these contributions can result in an increase or decrease in ferroelectricity with doping.


 Please wait while we load your content...
Please wait while we load your content...