Nonvolatile organic field-effect transistor memory from pyrene-fused azaindacene regioisomers†
Abstract
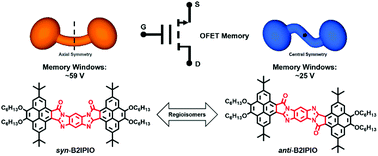
Nonvolatile memory devices based on organic materials are promising for a new generation of portable or flexible electronics. Herein, two pyrene-fused azaindacene configurational isomers, syn-B2IPIO and anti-B2IPIO, were designed and synthesized, and they are axially symmetric and centrally symmetric, respectively. These two regioisomers were applied as charge trapping elements (CTEs) for the electret layers in organic field-effect transistor nonvolatile memory (OFET-NVM) devices. Although the two regioisomers have nearly identical molecular structures, absorption spectra, and HOMO/LUMO energy levels, they exhibit distinctly different charge-trapping capabilities. The OFET-NVM devices based on anti-B2IPIO exhibit a moderate memory window of about 25 V. In contrast, the devices based on syn-B2IPIO show a more than two-fold wider memory window (∼59 V). The different charge trapping behaviors of this pair of isomers are attributed to different intermolecular interactions associated with molecular symmetry, resulting in different molecular packing behaviors and film morphologies. The results reveal that controlling the molecular symmetry could be a new and efficient strategy for the design of organic CTEs for high-performance memory devices.


 Please wait while we load your content...
Please wait while we load your content...