Gallic acid capped Tb3+-doped CaF2 nanocrystals: an efficient optical probe for the detection of carbonate and bicarbonate ions†
Abstract
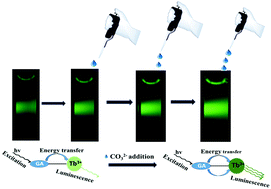
Detection of carbonate and bicarbonate ions in an aqueous medium is important due to their significance in diverse fields like environmental, materials and life sciences. In this work, we have designed a luminescence resonance energy transfer-based method for the detection of carbonate and bicarbonate ions. This is achieved using gallic acid-capped CaF2:Tb3+ nanocrystals, where gallic acid (GA) plays a multifunctional role. In addition to controlling the size as a capping molecule, it acts as a sensitizer to enhance the luminescence from Tb3+ ions as well as a linker molecule between the nanocrystals and the analytes (carbonates and bicarbonates). The gallic acid-capped CaF2:Tb3+ luminescent probe displays strong green emission at 544 nm (excitation at 296 nm) through sensitization of GA ligands. Further increase in the intensity of the green emission is noted upon the addition of carbonate and bicarbonate ions. This enhancement in the Tb3+ emission intensity is very selective and devoid of any interference from several other similar analytes. The calculated detection limit values are 0.99 μM, 1.3 μM, 1.23 μM and 2.15 μM for Na2CO3, K2CO3, (NH4)2CO3 and NaHCO3, respectively (by applying the 3σ/K criterion). In addition, a good linear range in the detection is noted for both carbonate and bicarbonate ions. Finally, the detection efficiency of the nanocrystal probe is verified with real-world water samples as well as with human blood samples.


 Please wait while we load your content...
Please wait while we load your content...