Elucidating the growth mechanism of ZnO films by atomic layer deposition with oxygen gas via isotopic tracking†
Abstract
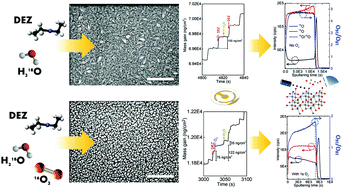
The growth process of zinc oxide (ZnO) thin films by atomic layer deposition (ALD) accompanied by the presence of oxygen gas pulsing is investigated by means of the isotopic tracking of oxygen 18O from the water precursor and oxygen 16O from the gas. In a previous study [T. Nguyen et al., Results Mater., 2020, 6, 100088, DOI: 10.1016/j.rinma.2020.100088], by means of structural, electrical, and optical characterizations, we identified key growth parameters of this unusual ALD process. Unexpectedly, the influence of molecular oxygen on the crystallography, microstructure, and morphology of the hundred-nanometer- to micrometer-thick ZnO films was significant. In this study, we present an unprecedented methodology by combining isotopic tracers with mass spectrometry to elucidate the role of the two different sources of oxygen atoms during the evolution of the growth. Notably, the use of in situ quartz crystal microbalance (QCM) and Secondary Ion Mass Spectrometry (SIMS) reveals new insights into the reaction mechanism for ZnO thin film growth. On the one hand, the non-negative mass change during the ZnO growth without O2 gas is attributed to the presence of bare zinc atoms on the surface due to the reaction between monoethyl zinc and hydroxyl groups of the water precursor after the diethyl zinc pulse. On the other hand, the detection of ZnxOyC2H5− ions by Time-of-Flight SIMS (TOF-SIMS) and the mass increase during the O2 pulse suggest a new reaction mechanism for the ZnO thin film growth in the presence of gaseous O2 where the ethyl ligand of the zinc precursor can react with O2 to form ethylperoxy radicals. The formations of the ethylperoxy zinc and/or zinc atoms lead to more adsorption of water to form ethylhydroperoxide during the water pulse, inducing the positive mass change. The use of an isotopic substitution allowed us to unambiguously associate the mass gain with the gradual incorporation of gaseous oxygen throughout the growth process and thereby support the chemical reaction.


 Please wait while we load your content...
Please wait while we load your content...