Electronic and crystal structure of bi-intercalated titanium diselenide CuxNiyTiSe2†
Abstract
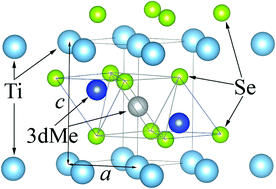
A comprehensive study of the crystal and electronic structure of TiSe2 intercalated with two 3d metals, Cu and Ni, is presented. It is found that the intercalation of Cu into NixTiSe2 at room temperature leads to the displacement of the Ni atoms from the octahedral to tetrahedral sites with respect to the Se sublattice. This effect is explained by the charge transfer from Cu to the TiSe2-derived conduction band, which makes the chemical bond of Ni with the lattice more stable. This is because the hybridization between Ni 3d and Se 4p, 4s states is stronger than that between Ni 3d and Ti 3d in mono-intercalated NixTiSe2. Therefore, it is possible to control the type of the coordination of the intercalated transition metal atom by changing the concentration of doped electrons.


 Please wait while we load your content...
Please wait while we load your content...