Thermal crystallization of amorphous calcium phosphate combined with citrate and fluoride doping: a novel route to produce hydroxyapatite bioceramics†
Abstract
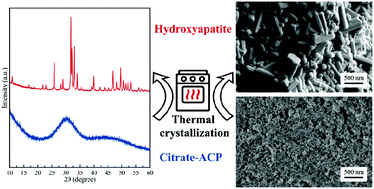
Amorphous calcium phosphate (ACP) is a material of high interest for dentistry, orthopedics, and other biomedical sectors. Being intrinsically metastable, the process of transformation of ACP into a crystalline phase upon heating is of high relevance for the development of innovative bioceramics. Here we have first studied the thermal behavior of a citrate-stabilized ACP (Cit-ACP) also doped with fluoride ions (Cit-FACP) prepared at three different nominal Cit/Ca ratios (i.e. 4, 2, 1) by differential thermal analysis. Next, the physico-chemical features of the crystalline products as well as the in vitro cell response to the materials were investigated. A citrate and fluoride free ACP sample was also tested as the blank. We have found that the activation energy of crystallization of Cit-(F)ACP samples is lower in comparison to the blank ACP and this is influenced by the nominal Cit/Ca molar ratio. Interestingly, we have discovered that the thermal treatment of Cit-(F)ACP at 800 °C yields hydroxyapatite (HA) or fluorapatite (FHA) as the main products differently from blank ACP that, like most of the ACPs reported in the literature, yields β-tricalcium phosphate. This was attributed to the Ca/P ratio of Cit-(F)ACP, which is similar to HA. A study of the crystalline products has revealed that all the (F)HA samples were non-cytotoxic, and retained carbonate ions in the crystal structure despite the heat treatment that should have induced decarbonation. The morphology of the products is influenced by the nominal Cit/Ca ratio and the presence of fluoride, ranging from spherical nanoparticles to micrometric hexagonal rods. Overall, our results prove that the thermal crystallization of Cit-(F)ACP is markedly different from classic ACP based materials and the thermal treatment of Cit-(F)ACP represents an attractive route for producing pure bioactive HA ceramics.


 Please wait while we load your content...
Please wait while we load your content...