Chemodynamic/photothermal synergistic therapy based on Ce-doped Cu–Al layered double hydroxides†
Abstract
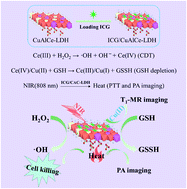
The combination of chemodynamic therapy (CDT) with photothermal therapy (PTT) is an efficacious strategy in cancer treatment to acquire satisfactory therapy efficiency in the endogenous redox reaction and external laser induction. In this work, we have designed Ce doped Cu–Al layered double hydroxide (CAC-LDH) ultrathin them through a bottom-up synthesis method, and further loaded them with indocyanine green (ICG). The synthesized ICG/CAC-LDH was used as a Fenton-catalyst and photothermal agent. With the Fenton activity, the ICG/CAC-LDH nanosheets could decompose H2O2 and exhibit a low KM value (1.57 mM) and an ultra-high Vmax (4.88 × 10−6 M s−1) value. Due to the presence of oxidized metal ions, ICG/CAC-LDH could induce intracellular GSH depletion and reduce Cu2+ and Ce4+ to Cu+ and Ce3+, respectively. The generated Cu+ and Ce3+ further reacted with local H2O2 to generate toxic hydroxyl radicals (˙OH) via the Fenton reaction. Owing to the obviously enhanced absorption of ICG/CAC-LDH at 808 nm, the photothermal efficiency of ICG/CAC-LDH increased significantly compared with ICG (ΔT = 34.7 °C vs. 28.3 °C). In vitro studies substantiate the remarkable CDT/PTT efficacy, with complete apoptosis of HepG2 cancer cells (the cell viability is less than 2%) treated with 25 μg mL−1 of ICG/CAC-LDH. Furthermore, ICG/CAC-LDH could also act as a contrast agent for cancer magnetic resonance imaging (MRI) and photoacoustic imaging (PAI). These results demonstrate the potential of ICG/CAC-LDH as an integrated agent for dual-modal imaging and synergistic CDT/PTT.


 Please wait while we load your content...
Please wait while we load your content...