One-pot, self-catalyzed synthesis of self-adherent hydrogels for photo-thermal, antimicrobial wound treatment†
Abstract
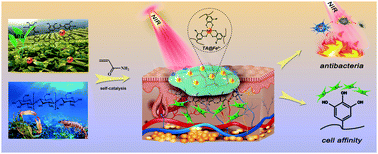
Self-adhering hydrogels are promising materials to be employed as wound dressings, because they can be used for wound healing without the necessity of additional stitching. However, micro-organisms can easily adhere to these hydrogels as well, which usually causes wound infections. Therefore, adhesive hydrogels are often combined with antibiotics. However, this introduces a risk of drug resistance, cytotoxicity and poor cell affinity. Consequently, recently, there has been great interest in developing non-antibiotic, antibacterial adhesive hydrogels. In this article, we present a simple one-pot synthesis procedure to prepare self-adhesive hydrogels composed of poly(acrylamide) (PAM), naturally derived chitosan (CS) and tannic acid/ferric ion chelates (TA@Fe3+). TA@Fe3+ enables self-catalysis of the polymerization reaction. In addition, due to its near infrared (NIR) photothermal responsiveness, TA@Fe3+ allows for eliminating the bacterial activity with up to 91.6% and 94.7% effectivity against Escherichia coli and Staphylococcus aureus, respectively. Mechanical and adhesion testing shows that the hydrogels are tough as well as flexible and will adhere repeatedly to many types of biological tissues, which can be attributed to the combination of physical and chemical bonding between TA@Fe3+ and PAM and CS, respectively. Moreover, in vitro and in vivo tests indicate that the NIR photothermally active hydrogel can effectively prevent bacterial infection and accelerate tissue regeneration, which demonstrates that these hydrogels are promising functional materials for wound healing applications.


 Please wait while we load your content...
Please wait while we load your content...