Nitrogen-centered radical reaction leading to energetic materials: a mild and efficient access to N–N bridged compounds†
Abstract
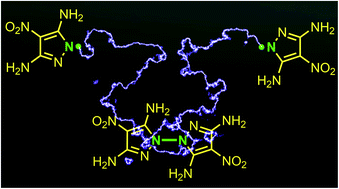
A radical reaction is a type of highly efficient organic reaction with a broad coverage in organic synthesis. However, its potential in the preparation of energetic materials is grossly underexplored. Now a novel nitrogen-centered radical reaction was discovered which features an N–N coupling capability, able to couple a pyrazole-based energetic compound to its N–N bridged dimer. The reaction exhibits high efficiency, while maintaining the traits of being mild, green, and facile without assistance from metal catalysis, photolysis, electrolysis or pyrolysis. The radical reaction mechanism was verified by a radical scavenger experiment and electron spin resonance spectroscopy.


 Please wait while we load your content...
Please wait while we load your content...