MXene binder stabilizes pseudocapacitance of conducting polymers
Abstract
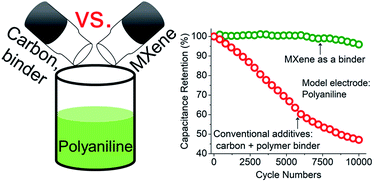
Conducting polymers (CPs) are by far the most studied organic materials for supercapacitors. Yet, their structural instability stemming from volumetric expansion/contraction during charge/discharge results in capacitance loss after moderate cycling that limits their applications. Here, we show that the remarkable cycling stability, capacitance, and rate performance can be achieved by replacing conventional electrode additives (carbon black or insulating polymer binder) with titanium carbide (Ti3C2Tx) MXene. Using polyaniline (PANI) as a model system, an addition of only 15 wt% of Ti3C2Tx MXene binder delivered remarkable capacitance retention of 96% after 10 000 cycles at 50 mV s−1 and high-rate capability with a capacitance of 434 F g−1. Using density functional theory (DFT) calculations, we show that, unlike insulating polymer binders, surface groups of MXene bond to PANI with a significantly high binding energy (up to −2.11 eV) via a charge transfer mechanism. This is one of the key mechanisms to achieve a high electrochemical performance of the CP-based electrodes when MXene is used as a binder. We expect that a similar approach can be used for stabilizing other organic electrode materials.


 Please wait while we load your content...
Please wait while we load your content...