Microstructural origin of selective water oxidation to hydrogen peroxide at low overpotentials: a study on Mn-alloyed TiO2†
Abstract
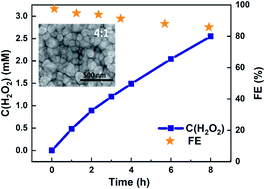
One key objective in electrocatalysis is to design selective catalysts, particularly in cases where the desired products require thermodynamically unfavorable pathways. Electrochemical synthesis of hydrogen peroxide (H2O2) via the two-electron water oxidation reaction (2e− WOR) requires a +0.54 V higher potential than four-electron O2 evolution. So far, best-performing electrocatalysts require considerable overpotentials before reaching peak faradaic efficiency. We present Mn-alloyed TiO2 coatings prepared by atomic layer deposition (ALD) and annealing as a stable and selective electrocatalyst for 2e− WOR. Faradaic efficiency of >90% at < 150 mV overpotentials was achieved for H2O2 production, accumulating 2.97 mM H2O2 after 8 hours. Nanoscale mixing of Mn2O3 and TiO2 resulted in a partially filled, highly conductive Mn3+ intermediate band (IB) within the TiO2 mid-gap to transport charge across the (Ti,Mn)Ox coating. This IB energetically matched that of H2O2-producing surface intermediates, turning a wide bandgap oxide into a selective electrocatalyst capable of operating in the dark. However, the high selectivity is limited to the low overpotential regime, which limits the system to low current densities and requires further research into increasing turn-over frequency per active site.
- This article is part of the themed collection: 2022 Journal of Materials Chemistry Lectureship shortlisted candidates


 Please wait while we load your content...
Please wait while we load your content...