Influence of diluent concentration in localized high concentration electrolytes: elucidation of hidden diluent-Li+ interactions and Li+ transport mechanism†
Abstract
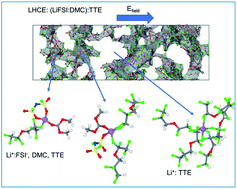
Localized high concentration electrolytes (LHCE) offer a viable dilution strategy for high concentration electrolytes (HCE) as the dilution process barely impacts the enhanced reductive/oxidative behavior of the HCE formulation but significantly lowers the overall viscosity and, in most cases, increases the ionic conductivity. On the other hand, experimental studies indicate that fluorinated ether electrolytes such as 1,1,2,2-tetrafluoroethylene 2,2,3,3-tetrafluoropropyl ether (TTE) help grow enhanced passivation layers on Ni-rich NMC cathodes. In this work, we study LHCE formulations based on lithium bis(fluorosulfonyl)imide (LiFSI), dimethyl carbonate (DMC), and TTE as the diluent. We use molecular dynamics methodologies, and Raman spectra measurements, to address to what extent the diluent content impacts the coordination behavior of the aggregated structures in the electrolyte and to evaluate the Li+ transport properties under the influence of an external electric field. In contrast to other fluorinated ethers, we find that TTE interacts with Li+via fluorine atoms, partially limiting the DMC–Li+ interactions hence altering the Li+ solvation coordination. This competitive interaction with Li+ between the organic solvent and the TTE diluent influences the electrolyte's reductive/oxidative behavior. Nevertheless, the bonding strength of the Li+–FTTE is much weaker than those of the Li+–ODMC and Li+–OFSI−. Therefore, the existence of Li+–FTTE is in a transient state rather than in a steady state. These results provide plausible guiding rules for future dilution strategies of HCE electrolytes. We also demonstrate that Li+ ions drift under the electric field's influence via repeated ion dissociation/association processes following a hopping conduction mechanism. Li+ ions jump between aggregated networks where Li–O interactions dominate via diluent-enriched phases, a process in which the solvation shells temporarily mutate to a Li–F dominated coordination structure. We expect our results to contribute an improved atomic-level understanding of the solvation structure and dynamics of LHCE electrolytes.


 Please wait while we load your content...
Please wait while we load your content...