Impact of hydration on ion transport in Li2Sn2S5·xH2O†
Abstract
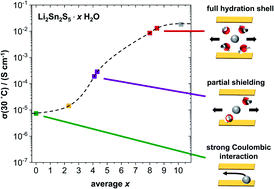
This work investigates the structure and transport properties of the layered material Li2Sn2S5·xH2O. The anhydrous phase shows a room-temperature Li+ diffusivity below 10−9 cm2 s−1 and conductivity below 10−5 S cm−1. Upon exposure to humidity, water intercalates between the layers and increases the interlayer distance, inducing first-order transitions to a hydrated phase (x ≈ 2–4) and then to a second hydrated phase (x ≈ 8–10). The latter is soft and sticky but remains solid. Diffusion of both Li+ ions and H2O remains predominantly two-dimensional under all conditions. The Li+ diffusivity and conductivity both increase by three orders of magnitude upon hydration, reaching values of 5 × 10−7 cm2 s−1 and 10−2 S cm−1 in the second hydrate. These transport rates are extraordinary for a solid electrolyte and approach what is typically seen in aqueous solutions. The material Li2Sn2S5·xH2O thus bridges the gap between a hydrated solid electrolyte and a confined liquid electrolyte, which is scientifically interesting and potentially useful in battery applications. In the light of these findings, a previous work on Li2Sn2S5 from our groups is revisited.


 Please wait while we load your content...
Please wait while we load your content...