Investigations of the stability of GaAs for photoelectrochemical H2 evolution in acidic or alkaline aqueous electrolytes†
Abstract
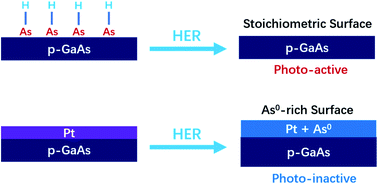
The long-term stability of p-GaAs photocathodes has been investigated for the hydrogen-evolution reaction (HER) in contact with either 1.0 M H2SO4(aq) or 1.0 M KOH(aq). Stability for the HER was evaluated using p-GaAs electrodes that were either etched or coated with active HER catalysts (Pt and CoP). Changes in surface characteristics of GaAs after exposure to electrochemical conditions were monitored by X-ray photoelectron spectroscopy (XPS), and electrode dissolution processes were evaluated by inductively coupled plasma mass spectrometry (ICP-MS). Consistent with thermodynamic predictions, after operation of the HER at pH 0 or pH 14, illuminated etched p-GaAs electrodes exhibited minimal dissolution while preserving a nearly stoichiometric surface. Electrodeposition or sputtering of Pt on the p-GaAs surface promoted the formation of excess As0via an interfacial reaction during the HER. The resulting non-stoichiometric As0-rich surface of p-GaAs/Pt electrodes caused a loss in photoactivity as well as substantial cathodic dark current. In contrast, p-GaAs electrodes coated with thin-film CoP catalysts did not display an increase in surficial As0 after operation of the HER in acidic electrolytes. Minimization of deleterious interfacial reactions is thus critical to obtain extended stability in conjunction with high performance from p-GaAs photocathodes.


 Please wait while we load your content...
Please wait while we load your content...