Highly dispersed Pt atoms and clusters on hydroxylated indium tin oxide: a view from first-principles calculations†
Abstract
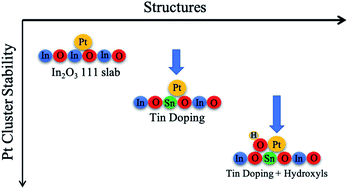
Supported single-atom and small cluster catalysts have become highly popular in heterogeneous catalysis. These catalysts can maximize the metal atom utilization while still showing superior catalytic performance. One of the main challenges in producing these small cluster catalysts is their low binding strength with the support, which causes these small clusters to sinter into larger nanoparticles. We have used first-principles simulations to study small Ptn (n: 1,2,3) clusters on indium oxide, tin doped indium oxide, and hydroxylated tin doped indium oxide. We report that the Ptn cluster is stabilized in the presence of tin and that this is especially the case for Pt single atoms on the hydroxylated indium tin oxide support, which are anchored to the support via the hydroxyl group. On this support, the Pt single atoms become more stable than Pt2 and Pt3 clusters, hence decreasing sintering. These findings provide a promising way to design single-atom catalysts on electrically conducting supports for electrocatalytic applications and to better understand how functional groups on supports can increase the adhesion of cluster catalysts.


 Please wait while we load your content...
Please wait while we load your content...