Oxygen reduction reaction over (Ba,Sr)6RE2Co4O15–Ba(Ce,Pr,Y)O3 composite cathodes for proton-conducting ceramic fuel cells†
Abstract
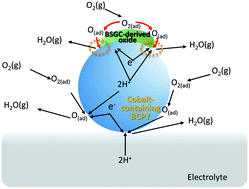
In this study, the effect of elemental substitution, especially the Ba/Sr ratio and rare-earth elements, in (Ba,Sr)6RE2Co4O15 (RE = La, Pr, Nd, Sm, Gd) and the composite effect with BaCe0.5Pr0.3Y0.2O3−δ on the activity for the oxygen reduction reaction were studied to develop high-performance cathodes for proton-conducting ceramic fuel cells. The polarization resistance of (Ba6−xSrx)RE2Co4O15 electrodes decreased with an increase in the Ba/Sr ratio, while the activity did not change systematically along the periodic table when the rare-earth element was substituted. Although the polarization resistance of Ba5SrGd2Co4O15 was about one order of magnitude lower than that of Ba4Sr2Sm2Co4O15 at 500 °C, the composite of Ba5SrGd2Co4O15–BaCe0.5Pr0.3Y0.2O3−δ (30 : 70 wt%) exhibited comparable performance and activation energy to Ba4Sr2Sm2Co4O15–BaCe0.5Pr0.3Y0.2O3−δ (30 : 70 wt%): polarization resistance – 0.20 Ω cm2 and 0.52 Ω cm2 at 600 °C and 500 °C, respectively, and activation energy – 61.8 kJ mol−1. Then, the reason for the high performance of the Ba5SrGd2Co4O15–BaCe0.5Pr0.3Y0.2O3−δ (30 : 70 wt%) composite was studied in detail, especially from the viewpoint of elemental interdiffusion. Finally, the plausible oxygen reduction reaction mechanism on this composite was proposed.


 Please wait while we load your content...
Please wait while we load your content...