pH-dependent hydrogen evolution using spatially confined ruthenium on hollow N-doped carbon nanocages as a Mott–Schottky catalyst†
Abstract
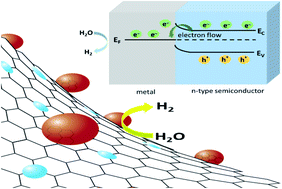
The hydrogen evolution reaction (HER) is known to exhibit pH-dependent kinetics that worsens with increasing pH. However, the HER in alkaline media is an integral part of regenerative alkaline fuel cells. Herein, an in situ pyrolysis strategy is adopted to obtain Ru decorated hollow N-doped carbon matrix (Ru@NCN) for pH universal HER. The synthetic approach is an advancement over traditional routes that use corrosive etchants like HF to achieve a hollow morphology for the carbon support. Ru@NCN shows superior HER in alkaline and neutral media relative to commercial Pt/C. To reach a current density of 10 mA cm−2, Ru@NCN required overpotentials of 36, 49, and 76 mV in alkaline, acidic, and neutral media, respectively. A correlation between the change in overpotential of Pt/C and Ru@NCN and pH is demonstrated. Additionally, the Mott–Schottky effect is observed at the metal/semiconductor (Ru/N-doped carbon) interface allowing facile electron transfer. It is envisioned that this report will provide a new direction to design hollow carbon nanostructures with an enhanced metal-support synergistic effect beneficial for sustainable energy conversion.
- This article is part of the themed collection: Showcasing recent research in materials chemistry from IIT Bombay, IIT Indore and IISc


 Please wait while we load your content...
Please wait while we load your content...