A novel amorphous P4SSe2 compound as an advanced anode for sodium-ion batteries in ether-based electrolytes†
Abstract
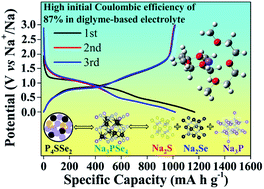
Anode materials with superior electrochemical performance are urgently needed for the development of sodium (Na)-ion batteries (NIBs). Ternary phosphides can deliver better electrochemical performance than binary counterparts due to the synergistic effect created by their multinary components. However, ternary phosphides can suffer from severe volume expansion during cycling due to their high crystallinity, leading to poor cycle performance. Here we, for the first time, synthesize the amorphous ternary phosphorus chalcogenide P4SSe2 compound, via a facile and scalable high energy ball milling method. This amorphous P4SSe2 compound shows great tolerance to volume expansion and fast kinetics, owing to its stepwise reaction and intermediate electrochemical products with superior conductivity. When applied as an anode for NIBs, the as-synthesized P4SSe2 delivers a reversible specific capacity of 1038 mA h g−1 with an initial coulombic efficiency up to 87%. Additionally, it shows more durable cycle life in the diglyme electrolyte than that in the EC:DEC electrolyte, which can be attributed to favorable molecular energy levels and stronger solvation energy, as confirmed by density functional theory calculations and X-ray photoelectron spectroscopy depth profile experiments, thus leading to the formation of an inorganic inner layer in solid electrolyte interphase films and preventing electrode material degradation from continuous side reactions with the electrolyte. Broadly, this study may excite more research interest in the synthesis of amorphous ternary phosphides and their application in the energy storage field.


 Please wait while we load your content...
Please wait while we load your content...