Storage mechanism of K in hydrogen-substituted graphdiyne as a superior anode†
Abstract
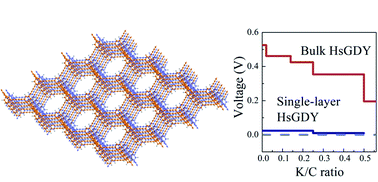
Potassium ion batteries (KIBs) have attracted great attention as promising alternatives to traditional lithium ion batteries. Seeking a proper anode material is a great challenge, due to the larger size of the K ion. We have proposed hydrogen-substituted graphdiyne (HsGDY) as the potential anode of KIBs by performing first principles calculations with molecular dynamic simulations. The adsorption mechanism and electrochemical properties of single-layer and bulk HsGDY were analyzed. With K-loading, HsGDY becomes a conductor with good conductivity along the stacking direction of the carbon layers. We found that no clusters of K were formed at high concentrations of K, due to the special adsorption mechanism around the edge of the holes in HsGDY. The specific capacity due to the K intercalation can reach 1094 mA h g−1 with a volume expansion of less than 30%. There was also a proper voltage profile with an average open circuit voltage of 0.38 V. The diffusion rate along the direction of the stacking layers was incredibly fast, with a diffusion barrier of 0.14 eV. The storage of K in HsGDY with a high concentration and a fast diffusion rate with high thermodynamic stability demonstrates the potential ability of HsGDY to be used as an excellent anode of KIBs.


 Please wait while we load your content...
Please wait while we load your content...