Study on solid electrolyte catalyst poisoning in solid acid fuel cells†
Abstract
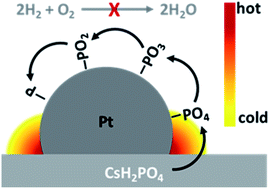
Solid acid fuel cells operate at intermediate temperatures utilizing a solid electrolyte (CsH2PO4, CDP). However, relatively little is known about the degradation mechanism and the topic is rarely addressed. Phosphate poisoning of the platinum catalyst is a well-known problem for fuel cells working with H3PO4 as electrolyte. With CsH2PO4 as electrolyte, phosphate poisoning is therefore likely to occur as well. In this study we show a fast and reversible degradation behavior of solid acid fuel cells and associate it with poisoning of the catalyst. After a decline in power output of around 50% within hours, an in situ reactivation of the cell to almost the initial performance was possible by multiple cycling between the voltage of 0.1 V and 2.0 V. A limitation of the effect to the cathode is shown and the underlying process was analyzed by changes in the low frequency domain of impedance measurements, which is indicating a catalyst poisoning, and by the dependency from the upper vertex voltage. By employing a micro porous current collector, a decrease in the low frequency domain as well as enhanced stability (<125 μV h−1 at 0.43 V) was achieved. This work extends from a detailed insight in the degradation mechanism of solid acid fuel cells, to providing a working electrode modification to prevent poisoning, establishing a promising electrode stability on a laboratory scale.


 Please wait while we load your content...
Please wait while we load your content...