Sodium difluorophosphate: facile synthesis, structure, and electrochemical behavior as an additive for sodium-ion batteries†
Abstract
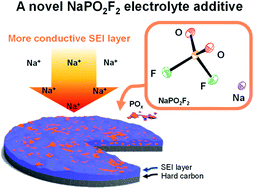
Despite the success of difluorophosphate (PO2F2−, DFP) electrolyte additives in lithium and potassium-ion batteries, their utilization in sodium-ion batteries remains unexplored due to difficulties in the synthesis of sodium difluorophosphates (NaDFP). Thus, in this study, NaDFP salt prepared via ion exchange of KDFP and NaPF6 is characterized using single-crystal X-ray diffraction, Raman and infrared (IR) spectroscopy, energy dispersive X-ray analysis (EDX), and thermogravimetry-differential thermal analysis (TG-DTA). Electrochemical tests demonstrate enhanced cycle performance of a hard carbon electrode (capacity retention; 76.3% after 500 cycles with NaDFP vs. 59.2% after 200 cycles in the neat electrolyte), achieving a high coulombic efficiency (average of 99.9% over 500 cycles) when NaDFP is used as an electrolyte additive. Further, electrochemical impedance spectroscopy (EIS) using a HC/HC symmetric cell demonstrates significant reduction of the interfacial resistance upon addition of NaDFP. X-ray photoelectron spectroscopy (XPS) indicates presence of stable, Na+-conducting solid-electrolyte interphase (SEI) components formed in the presence of NaDFP. This work not only presents a feasible NaDFP synthesis method, but also demonstrates the use of NaDFP as a strategy for optimizing sodium-ion battery performance.


 Please wait while we load your content...
Please wait while we load your content...