WC and cobalt nanoparticles embedded in nitrogen-doped carbon 3D nanocage derived from H3PW12O40@ZIF-67 for photocatalytic nitrogen fixation†
Abstract
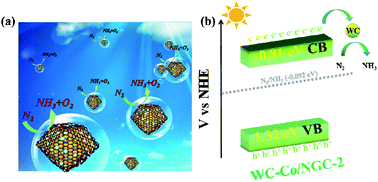
Ammonia is a momentous raw chemical in modern life; however, the application of green and environmentally friendly ways to manufacture ammonia remains an enormous dilemma that requests to be conquered at this stage. In this study, we employed H3PW12O40 (PW12) encapsulated with ZIF-67 as the precursor, and calcinated it at high temperatures in an N2 atmosphere to synthesize nitrogen-doped graphitic carbon (NGC) nanocages hybrid loaded with WC nanoparticles and Co nanoparticles (WC–Co/NGC). The NH3 formation rates of WC–Co/NGC-2 with the most palmary photocatalytic effect under visible light and simulated sunlight were 142 μmol g−1 h−1 and 157 μmol g−1 h−1, respectively. The formation rates approach six-fold higher than the individual Co/NGC due to WC–Co/NGC synthesizing after calcination and preserve as a 3D configuration. The specific surface area of WC–Co/NGC-2 far transcends that of Co/NGC, and the pore size distribution manifests many mesoporous structures, which can significantly increase the catalytic process and promote the reaction. Furthermore, the content of pyridine N in WC–Co/NGC-2 is higher than Co/NGC and WC, which can effectively serve as an electron operation center to assist the photocatalytic reaction, reduce the recombination of electrons and holes, and establish an eye-catching catalytic effect. This work not only synthesized WC–Co/NGC with facile tactics but also emerged a brand new speculating orientation for expanding the territory of photocatalytic nitrogen fixation materials.


 Please wait while we load your content...
Please wait while we load your content...