A ring-locking strategy to enhance the chemical and photochemical stability of A–D–A-type non-fullerene acceptors†
Abstract
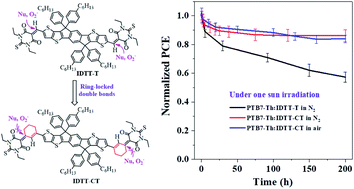
Recently, the power conversion efficiencies (PCEs) of bulk-heterojunction organic solar cells (BHJ-OSCs) based on non-fullerene acceptors (NFAs) have made a very impressive progress in the research field. However, less attention has been paid to the intrinsic chemical and photochemical stability of NFAs, although they are correlated greatly with the resulting device stability. Herein, we describe a new molecular design strategy to enhance the intrinsic chemical and photochemical stability of acceptor–donor–acceptor (A–D–A)-type NFAs by introducing ring-locked carbon–carbon double bonds between D–A conjugation, attributed to increased steric hindrance of nucleophilic attack and the formation of intramolecular C–H⋯O interactions. Based on this strategy, two types of NFAs were successfully prepared, 2-(1,1-dicyanomethylene)rhodanine-based IDT-CR and IDTT-CR and thiobarbituric acid-based IDT-CT and IDTT-CT. When blended with a wide-bandgap polymer donor (P3HT), the IDTT-CR-based solar cells can exhibit a PCE of 2.86%. Moreover, a much enhanced PCE of 6.13% was realized by adopting a low-bandgap polymer donor PTB7-Th to pair with IDTT-CT. The fabricated PTB7-Th:IDTT-CT-based OSCs showed very encouraging photostability, the PCE of which could retain >80% of the initial values after 200 h one sun irradiation in air without a UV filter. Such photostability performance has greatly outperformed those from conventional NFAs like ITIC, IT-4F, and IT-M, suggesting the effectiveness of our ring-locking design strategy. Moreover, PTB7-Th:IDTT-CT-based OSCs could retain ∼70% of its initial PCE after heating at 85 °C for 100 h. Furthermore, we reported an inferior device stability for P3HT:IDTT-CR based OSCs, which is primarily attributed to the evolution of BHJ film morphology under light illumination.


 Please wait while we load your content...
Please wait while we load your content...