Structure and chemistry of the solid electrolyte interphase (SEI) on a high capacity conversion-based anode: NiO†
Abstract
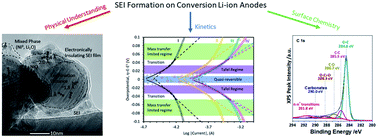
Electroanalytical techniques are specialized tools with high-sensitivity that when combined with electron transfer theory can shed light on the mechanisms of highly complex, heterogeneous, multi-step reactions. This study focuses on competitive reactions between the formation of the solid-electrolyte interphase (SEI) and the conversion reaction of a NiO Li-ion metal oxide anode as a function of state-of-charge (SOC). To our knowledge, this work is the first thorough analysis for the formation of the SEI on a conversion-based anode. NiO oxide was chosen because of recent reports in half cells and full cells showing good high-rate performance, though it is believed that NiO may act as a representative material for many of the conversion anodes. Galvanostatic intermittent titration technique (GITT) is applied to probe both the complex diffusional processes and the electrokinetic phenomena along the reaction pathway. Butler–Volmer (BV) and Marcus–Hush–Chidsey (MHC) models are used to investigate the reaction transfer coefficients and reorganizational energies at the inner/outer Helmholtz plane of the electrode/electrolyte interface. Next, the effective transfer coefficient is extracted and analyzed to provide new mechanistic insight into the rate determining step and the reaction pathway at different SOC.


 Please wait while we load your content...
Please wait while we load your content...