Incorporation of nickel single atoms into carbon paper as self-standing electrocatalyst for CO2 reduction†
Abstract
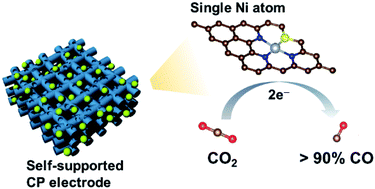
The design of selective and efficient catalysts for electrochemical CO2 reduction is highly desirable yet still challenging, in particular, if the aim is to make them binder-free and self-standing. Here, we report a new and straightforward strategy to incorporate Ni single atoms into a commercially available carbon paper to prepare a self-standing electrode. This is accomplished by consecutive acid activation, adsorption of Ni2+ ions, and pyrolysis steps. Structural characterizations and calculations based on density functional theory consistently suggest that the Ni single atoms are coordinated with three N and one S atoms on the carbon paper. When used for CO2 electroreduction, the electrode exhibits an optimal selectivity (91%), activity (3.4 mA cm−2), and stability (at least 14 h) for CO production in water at an overpotential of 660 mV. This report may inspire the design and incorporation of single atoms of various metal types into carbon papers, or other kinds of carbon substrates, for a wide range of electrocatalytic processes.


 Please wait while we load your content...
Please wait while we load your content...