Mechanism and equilibrium thermodynamics of H- and J-aggregate formation from pseudo isocyanine chloride in water†
Abstract
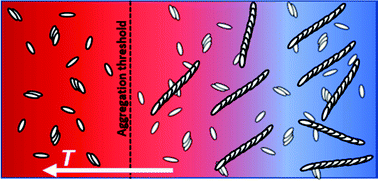
Pseudo isocyanine chloride (PIC) has a strong tendency to self-assemble with a concentration dependent temperature threshold separating a regime with small H-oligomers in equilibrium with monomeric PIC from a regime where large J-aggregates form. In complementing the known set of absorption spectra by the spectrum of a trimer, which represents all H-aggregates with N ≥ 3, a full description of the sample composition of PIC in the regime of oligomers became possible by means of UV-vis spectroscopy and gave access to the equilibrium thermodynamics of oligomerisation. Successful interpretation of the concentration dependent temperature threshold as a ceiling temperature of J-aggregation made also accessible equilibrium thermodynamics of the formation of J-aggregates together with a full analysis of composition also in the regime of J-aggregates. Evolution of an invariant spectrum for the J-aggregates demonstrates full consistency of the composition analysis. Complementary light scattering experiments led to a comprehensive characterisation of all aggregate species above and below the aggregation threshold. Once initiated, J-aggregates always grow to a final length of 650 nm. Time-resolved light scattering confirmed that the self-assembly of J-aggregates follows a monomer addition process in analogy to a chain growth in polymer chemistry. Initiation and growth of individual aggregates turned out to be always much faster than the progress of aggregation.


 Please wait while we load your content...
Please wait while we load your content...