Operation of calcium-birnessite water-oxidation anodes: interactions of the catalyst with phosphate buffer anions†
Abstract
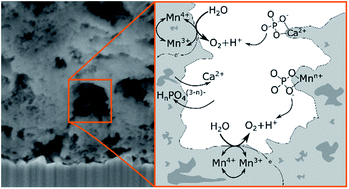
Investigating the interfaces between electrolytes and electrocatalysts during electrochemical water oxidation is of great importance for an understanding of the factors influencing catalytic activity and stability. Here, the interaction of a well-established, nanocrystalline and mesoporous Ca-birnessite catalyst material (initial composition K0.2Ca0.21MnO2.21·1.4H2O, initial Mn-oxidation state ∼+3.8) with an aqueous potassium phosphate buffer electrolyte at pH 7 was studied mainly by using various electron microscopy and X-ray spectroscopy techniques. In comparison to electrolyte solutions not containing phosphate, the investigated Ca-birnessite electrodes show especially high and stable oxygen evolution activity in phosphate buffer. During electrolysis, partial ion substitutions of Ca2+ by K+ and OH−/O2− by HnPO4(3−n)− were observed, leading to the formation of a stable, partially disordered Ca–K–Mn–HnPO4–H2O layer on the outer and the pore surfaces of the active electrocatalyst material. In this surface layer, Mn3+ ions are stabilized, which are often assumed to be of key importance for oxygen evolution catalysis. Furthermore, evidence for the formation of [Ca/PO4/H2O]− complexes located between the [MnO6] layers of the birnessite was found using the soft Ca 2p and Ca L-edge X-ray spectroscopy. A possible way to interpret the observed, obviously very favorable “special relationship” between (hydrogen)phosphates and Ca-birnessites in electrocatalytic water oxidation would be that HnPO4(3−n)− anions are incorporated into the catalyst material where they act as stabilizing units for Mn3+ highly active centers and also as “internal bases” for the protons released during the water-oxidation reaction.


 Please wait while we load your content...
Please wait while we load your content...