Modeling the electrochemical behavior and interfacial junction profiles of bipolar membranes at solar flux relevant operating current densities†
Abstract
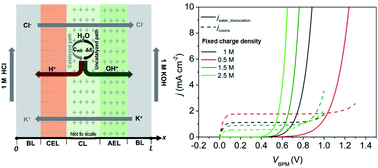
A 1-dimensional, multi-physics model that accounts for the migration and diffusion of solution species, electrostatics, and chemical reactions, in particular water dissociation (WD), at bipolar membrane (BPM) interfaces was developed to study the electrochemical behavior of bipolar membranes (BPMs) at solar flux relevant operating current densities (tens of mA cm−2). Significant partial current densities for WD were observed at BPM voltages much less than the equilibrium voltage, e.g., 59 mV × ΔpH from both experiments and modeling. The co-ion leakage across the BPM at pH differentials accounted for the early presence of the partial current density for WD. Two distinctive electric field dependent WD pathways, the un-catalyzed pathway and the catalyzed pathway, were quantitatively and parametrically studied to improve the turn-on potential of the BPM. The catalyzed pathway accounted for the majority of the partial current density for WD at low voltages, while the un-catalyzed pathway dominated the WD at relatively high voltages. Significant WD was observed only within the interfacial CL (<5 nm), in which a large electric field was present. To improve the electrochemical behavior and the turn-on potential of BPMs, the impacts of the pKa of the immobilized WD catalysts, the electric-field dependent rate constant, the thickness of the catalyst layer and fixed charge density in BPMs on the partial current densities for WD were studied systematically. In addition, the electrochemical behavior and concentration profiles of BPMs in a buffered electrolyte were studied and contrasted with those in an un-buffered electrolyte from both modeling and experiments.


 Please wait while we load your content...
Please wait while we load your content...