Investigating the stable operating voltage for the MnFe2O4 Li-ion battery anode†
Abstract
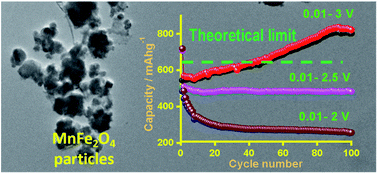
Template-free synthesis of MnFe2O4 nanopowder is carried out by co-precipitation in a basic medium, evaluating the effects of cation ratio and reaction temperature on the phase composition crystallinity of the resulting powder. Single-phase samples of the target spinel are obtained at the stoichiometric Mn : Fe = 1 : 2 ratio under reflux conditions (100 °C), as confirmed by X-ray diffraction (XRD) and Fourier Transformed Infrared (FTIR) spectroscopy. Transmission electron microscopy (TEM) images confirmed that nanostructured MnFe2O4 particles are obtained, which is further supported by Debye–Scherrer calculations from XRD data and by AFM measurements. The produced oxide demonstrated considerable thermal stability according to TGA data. Magnetic characteristics are strongly dependent on the content of magnetic phase and phase composition, achieving a maximum of 54 emu g−1 for single-phase stoichiometric MnFe2O4. Further, the electrochemical stability of this material as the anode is investigated in Li-ion batteries (LIBs). When the MnFe2O4 electrode is operated in the potential window of 0.01–3.0 V, the reversible capacity is enhanced by almost 45% (802 mA h g−1) after the 100th cycle with reference to the 2nd cycle reversible capacity (548 mA h g−1). Methodically dQ/dV plots are analyzed and compared to understand processes behind the evolution of extra capacity beyond its theoretical limit. Further, the upper cut-off potential is tuned to identify a stable operating potential window for the MnFe2O4 anode in LIBs.
- This article is part of the themed collection: Energy Frontiers: Electrochemistry and Electrochemical Engineering


 Please wait while we load your content...
Please wait while we load your content...