Reaping the catalytic benefits of both surface (NiFe2O4) and underneath (Ni3Fe) layers for the oxygen evolution reaction†
Abstract
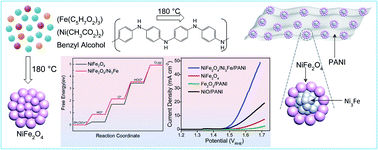
Surface and interface engineering predominantly determines the overall surface dynamics, and the surface layers can benefit substantially from a chemically different underneath layer. Herein, we demonstrate the surface tuning of nickel ferrite (NiFe2O4) with awaruite (Ni3Fe). The NiFe2O4 grows in the form of a crystalline monolayer encompassing bimetallic Ni3Fe nanocrystals embedded within a polyaniline (PANI) matrix. This is achieved by a simple and highly reproducible chemical approach. Benzyl alcohol (BA) and PANI steer the growth of metallic Ni3Fe covered with a monolayer of NiFe2O4 under mild solvothermal conditions. PANI presumably renders anchoring sites and guides the formation of fine and monodisperse nanocrystals of NiFe2O4/Ni3Fe, while BA acts as a reducing agent and controls the nucleation and the growth of NiFe2O4. In the absence of PANI, the formation of large and agglomerated NiFe2O4 nanoparticles is observed. The fine interface created between the NiFe2O4 monolayer and Ni3Fe imparts unique and superior surface attributes; Ni3Fe renders a high electroactive surface area and faster charge transport, while the NiFe2O4 monolayer affords high catalytic ability. As a result, the NiFe2O4/Ni3Fe/PANI electrode efficiently catalyzes the oxygen evolution reaction (OER), and requires a substantially lower overpotential than those of NiO/PANI, NiFe2O4, and Fe2O3/PANI. The Tafel values suggest that the kinetics of the half-reaction at the catalytic interface of NiFe2O4/Ni3Fe/PANI is much faster (52.3 mV dec−1) than that of NiO/PANI (82.5 mV dec−1), NiFe2O4 (86.0 mV dec−1) and Fe2O3/PANI (108.3 mV dec−1). The turnover frequency of NiFe2O4/Ni3Fe/PANI is 0.27 s−1, 5.4 fold higher than that of NiFe2O4 (0.05 s−1). Moreover, the electrochemically active surface area, electrochemical impedance, surface charge transport properties, and active sites of NiFe2O4/Ni3Fe/PANI and NiFe2O4 are estimated, compared and correlated to the OER performance. In addition, density functional theory calculations show a decrease in the barrier of the potential-determining step from *O to *OOH, indicating a favorable electronic modulation at the interface of NiFe2O4 and Ni3Fe, which reduces the overpotential requirement.


 Please wait while we load your content...
Please wait while we load your content...