Synthesis of amino alcohols, cyclic urea, urethanes, and cyclic carbonates and tandem one-pot conversion of an epoxide to urethanes using a Zn–Zr bimetallic oxide catalyst†
Abstract
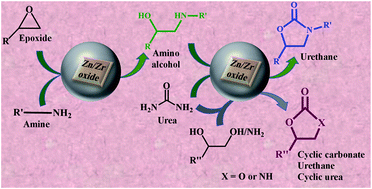
The insertion of CO produces useful chemicals such as urethanes, cyclic carbonates, and cyclic urea using CO2 or urea as a sacrificial source. Synthesis of these chemicals using CO2 as a reactant requires stringent conditions such as high pressure and temperature and complicated catalyst design. Similar products can be prepared using urea as a sacrificial CO source employing simple catalysts having optimum acidity and basicity. This study demonstrates urethane synthesis directly from an epoxide using a Zn–Zr bimetallic oxide catalyst in a one-pot tandem reaction. First, amino alcohols are synthesized using the Zn–Zr catalyst, and then the amino alcohols are reacted with urea to produce urethanes using the same catalyst. Moreover, cyclic urea and glycerol carbonate/other cyclic carbonates are prepared by the reaction of diamine or glycerol/diols with urea. Optimum amounts of Zn and Zr having optimum acidity and basicity are required to achieve the best catalytic activity in the individual steps and one-pot tandem conversion of an epoxide to urethanes. The catalyst is efficiently recyclable with retention of activity without losing the catalytically active phase and species. A simple, solvent-free, economical, and eco-friendly catalyst affording three important chemicals, using urea as a sacrificial reactant, would attract significant scientific and industrial interest.


 Please wait while we load your content...
Please wait while we load your content...